🔋Diamond In The Rough pt. 2
Both types of graphite production (synthetic and natural) as they stand are in direct contradiction to the mission of electric vehicles.
Help me out and press the heart button above or below I would greatly appreciate it!
Last week I discussed the incoming supply/demand imbalance in graphite, the geopolitical counterparty risk in terms of graphite processing, and some potential avenues that this predicament could go down. On top of that, the greenness of Li-ion batteries is debated because of the tremendous mineral mining requirements. EVs contribute less emissions than any other type of vehicle for the lifetime of the vehicle but there is an ‘emissions payback period’ through continued zero-emissions as the car is driven (if you crash it before ~13,000mi you didn’t help the environment). A further issue is the green energy movement is more or less at war against fossil fuel industry even though they are thoroughly dependent as I will explain.
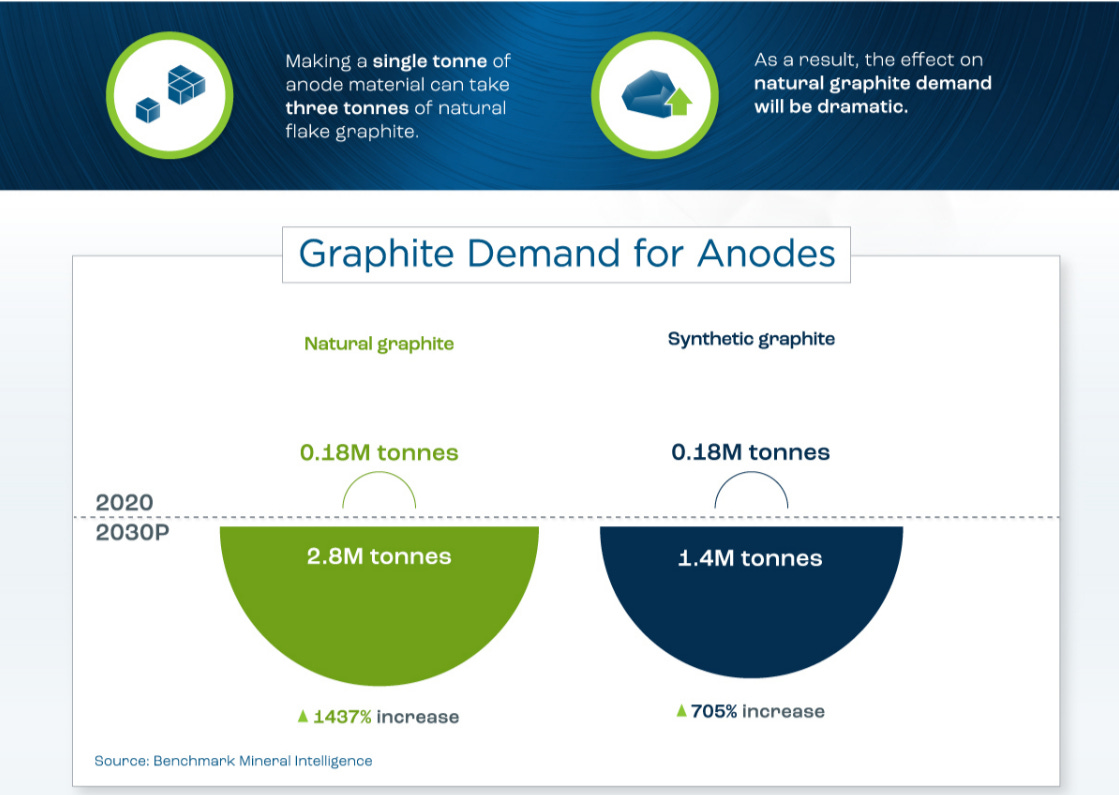
Graphite itself is pure carbon in layers of hexagonally oriented carbons, where the only difference to diamond is the structure. Last week I mentioned briefly there are two types of graphite used for Li-ion battery anodes, natural (NG) and synthetic (SG). SG remains the dominant version of battery-grade graphite but it is still fairly mixed. In the future NG is expected to be the dominant version, but both will increase in demand exponentially regardless of preference.
Synthetic Graphite
Synthetic graphite is formed through a transformation of petroleum coke or coal tar pitches. Petroleum coke is produced during the oil refining process and has many other industrial uses beyond just making graphite. From the national association of manufacturers,
Petroleum coke is a valuable and essential commercial product that is used directly in a wide range of applications including aluminum manufacturing, fuels, and numerous other products including steel, glass, paint, and fertilizers. Petroleum coke is also used as a fuel in power generation, cement kilns and other industries.
The graphitization of cokes occurs through a heat treatment at 3000°C for 2-3 hours. This can be accelerated with higher pressure, various metal catalysts, or oxidizing gases. This is a tremendously energy intensive process but is required to transform the initial coke into graphite of the right crystalline size and interdimensional spacing for battery anodes. The energy demand of this process is costly, which is a main reason for the increased interest in NG for battery anodes.
It goes without saying that since synthetic graphite is derived from fossil fuels and requires the burning of more in the graphitization process, that it in no way fits the current definition of green/clean.
Natural Graphite
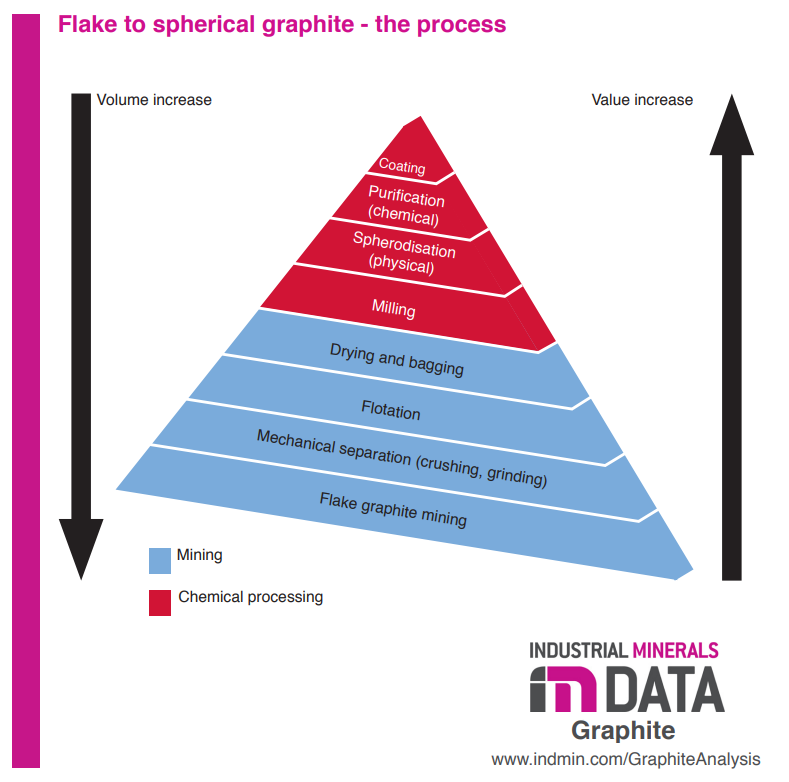
Natural graphite comes straight out of mineral deposits and is then refined into the correct size and purity required for batteries. This is considered flake or large flake graphite with >99% graphite content. The mined graphite rock is crushed and grinded, followed by a process called froth flotation where it separates hydrophobic (water resistant) from hydrophilic (mixes with water) minerals in water. After drying, the flake graphite goes for chemical processing where it will transform into spherical graphite for those desiring higher quality for battery anodes. After it is first milled to the desired size, spheroidization is an other physical step where the it is shaped into spheres which increase performance characteristics for Li-ion batteries. Next it is purified with either very high temperatures or a chemical process which includes strong acids. Coatings can be applied to further increase the performance/desirability for battery anodes.
Conclusion
Graphite is an essential material for the anode in Li-ion batteries and it has to be of extremely good quality in order for the best batteries to be put into new electric vehicles. Demand for graphite is projected to be high enough even synthetic graphite which is more expensive and contributes higher emissions. In fact, SG produces roughly 3x more carbon dioxide emissions than NG and is roughly twice as expensive. Even natural graphite is a harsh process environmentally.
However, the physical separation of graphite from its ore body is known to be very expensive, energy intensive and time-consuming. - Jara et al.
On top of this, a large percentage of China’s manufacturing is powered by coal which is where most graphite comes from regardless of type. Yes natural graphite will be preferably favored, but a 705% increase in synthetic is still expected. The production of this material directly contradicts the mission of the EV revolution and green movement. The US is currently okay with burning fossil fuels to use a fossil fuel biproduct to synthesize graphite and then import 100% of what it needs via transportation requiring fossil fuels into the country for electric vehicles whose sole purpose is to displace use of fossil fuels. Does the Machiavellian principle of the ends justifying the means apply here?
It may feel good to have an electric vehicle, but what goes into making it sure shouldn’t. China has had cheaper labor and lower environmental regulations which helped them gain outsized dominance in the market for graphite, cathode materials, and rare earth metals. It is with extreme difficulty that new mines could be started here in the United States for example. This is the classic example of ‘out of sight, out of mind’ where we have no problem letting other countries doing the dirty work to give us the things we want so we don’t have to see it.
Are there any solutions to this strange predicament? Graphite is by far the best anode for lithium ion batteries on the market. The idea behind solid-state batteries is to get rid of graphite all together in favor of a lithium or silicon based anode. I have talked in depth about solid-state batteries and their ability to not only solve the graphite problem, but to in theory increase charging speed, energy density, and safety. This is developing technology and may not engulf the battery market unless the product is better than Li-ion by a lot. Graphite mining AND processing closer to the end user is another way for emissions to be abated, albeit requiring immense capital investment. Developing more sustainable and energy efficient processes for graphite production could also help, which include switching from synthetic to natural like is already occurring.
At current stage, the emission goals of the current energy transition/green energy movement directly contradict the decisions made/process used to achieve their goal of 50% electric vehicles by 2030 for example. Yes, steps are being taken and companies are working diligently on solutions, but the path we are on does not take realistic estimates of these developments into account. The arbitrary targets set forth by major western nations like the US are causing a tremendous shock to resource demand as well as transforming capital allocations. Ironically enough, oil refineries produce the petroleum coke in order to make synthetic graphite. Unfortunately, the government is at war with oil refineries here in the US which impacts more than just gas prices. With them operating at ~94% capacity and no signs of opening any more major facilities (perhaps ever), this will certainly not help with the ~704% increase in synthetic graphite needed.
It is difficult to see how full battery electric vehicles are a solution when the US concurrently could not stand to see the mining/processing of batteries materials in our own country as well. This only reinforces the idea of a more realistic path forward with hybrids to abate the majority of emissions coming for cars while the new technologies and methods advance. All cars having a ~30mi electric range for example would be better than failing to achieve the EV target and having less than 50% of all new cars full electric in 8 years. Until next week,
-Grayson
Leave a like and let me know what you think!
If you haven’t already, follow me at twitter @graysonhoteling and check out my latest posts.
Let someone know about Better Batteries and spread the word!
Socials
Twitter - @graysonhoteling
LinkedIn - Grayson Hoteling
Email - betterbatteries.substack@gmail.com
Archive - https://betterbatteries.substack.com/archive
Subscribe to Better Batteries
Please like and comment to let me know what you think. Join me by signing up below.





Grayson, I would be interested on your thoughts about C-Si anodes. I am a bit out of touch, but understand that for some applications (not EVs yet), these anodes are feasible and possibly in use now ("wearables"?). Do you believe that the C-Si anode will make it into EVs, and if so , in what time frame? Thanks.