🔋No More Liquids
Are solid-state batteries the future of energy storage?
If you’re reading this, I would really appreciate it if you pressed the heart button above :)
The demand for lithium-ion batteries (LIBs) has been skyrocketing in recent years and seems to be at an inflection point. Electric vehicles, grid storage, satellites, consumer applications, and more seem to be ever increasing. Talk and worries about raw material shortages more than the actual ability to produce battery cells are looming over the industry. Be sure to check out my previous pieces on what batteries are made of, the big picture about electric vehicles (EVs), and what determines the price of batteries. These lead into my discussion here today for more context. Moreover, increasing demand for EVs has highlighted unfortunate safety concerns with LIBs. In reality, the biggest concerns with EVs as they stand are cost and charging time. So where do solid-state batteries (SSB) come into play, are they realistic, and what the heck are they?
Chemistry Stuff
I won’t go into the specifics of what materials there are as I want this to be a macro picture, not the nitty-gritty. Just know there are two categories, inorganic/ceramic and polymer. There are some fundamental properties that the solid electrolyte should abide by. The material chosen needs to be very ionically conductive (of Li ions), but also be an electronic insulator (not conduct electrons). The purpose of the electrolyte is to facilitate the travel of lithium ions back and forth from anode to cathode and vice versa. The solid material chosen cannot inhibit this fundamental process. The second piece about not conducting electrons is so that the electrons travel through the external circuit and do not flow directly to the other electrode causing a short circuit. Furthermore, the material chosen must itself be electrochemically stable within the operating voltage range and chemically stable with the anode and cathode materials as side-reactions can severely hamper battery performance or even ruin it. The material must be thermally stable to perform in an array of temperature environments. The materials themselves should also not be toxic and have safe and sustainable production methods.
Advantages
The complete and concise overview on the LIB system can be found in my ultimate battery guide. The fundamental feature of solid-state batteries is that they replace the liquid electrolyte with a solid one. What this means is that much of the existing infrastructure to produce LIBs will also be able to be used for solid-state batteries, as they aren’t a completely new technology. The story of solid-state batteries is really just the story of the solid electrolyte. Why is this necessary you might ask, my phone battery works just fine? Well, there are a few reasons…
First, the organic liquid electrolyte solvents used in all LIBs today are hazardous and flammable materials. There have been safety concerns over batteries catching fire and EV recalls due to faulty battery packs. Generally, faulty manufacturing and/or poorly managed temperature and depth of charge can cause thermal runaway and oxygen release. Adding a flammable liquid electrolyte to a system that undergoing thermal runaway (especially if there is some oxygen evolution inside as well) is clearly not ideal. A solid electrolyte is inherently safer and does not pose the same explosion/fire risk as traditional LIBs. This also means from a production standpoint, storage and handling of these volatile materials is not necessary.
Second, solid-state batteries could hold the key to alternative anodes. The lithium metal battery (lithium anode instead of graphite) has really been the unattainable holy grail for batteries since they were discovered (people can make the work, but none have been commercialized to large scale). Lithium metal offers a much higher energy density than the graphite anodes being used currently. Less lithium may be used compared to graphite meaning it can increase gravimetric capacity (charge stored per unit mass) and volumetric capacity (charge stored per unit volume). * This figure below shows just how much less volume the cells are where the same energy (perhaps more) is packed into a smaller space.
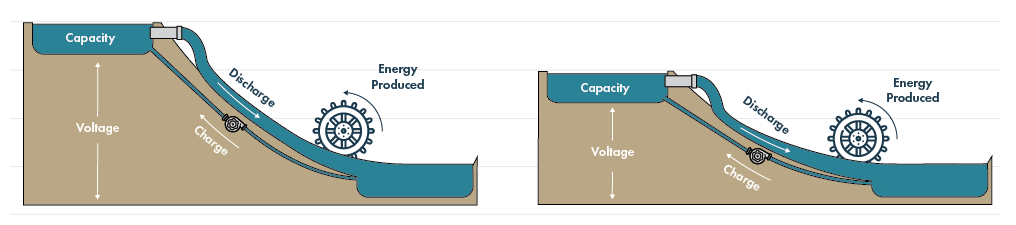
Perhaps more… With lithium as the reference point instead of graphite the operating voltage is about 100mV higher which increases the energy density (voltage x capacity per unit mass or volume). This is the real indication of how much juice is in the cell. The higher the voltage, the more energy produced with the same amount of capacity which can be visualized beautifully in this illustration below.
Another anode possibility is silicon anodes. Silicon is a good option due to its high natural abundance in the earth and its high capacity like lithium metal leading to higher energy density compared to graphite as well. It has a suitable voltage which is less ideal than Li metal, but better than graphite. The issues with silicon anodes are volume expansion, poor electronic conductivity, and reactions with liquid electrolytes. Finding a suitable solid electrolyte could eliminate the issue with electrolyte reactions and reduce the impact of volume expansion.
Charge time is another possible draw to solid state batteries. No one wants to wait forever to charge their car to keep driving. This needs to be as painless or easier than filling up a tank of gas. I have seen plenty of people say they won’t get an EV based off this factor alone. Currently it takes longer to charge a car than to fill up a tank of gas. Even though traditional fast charging of LIBs has gotten very fast (down to 15min), it still poses challenges such as decreased capacity retention, lithium plating issues, and high electrochemical impedance. Lithium metal batteries with a solid electrolyte actually use lithium plating to their advantage. Unfortunately, capacity retention, impedance at interfaces, and other performance factors still have to be dealt with before they are commercialized [1].
Disadvantages
The commercial target for a fast-charging system is to maintain 80% discharge energy over 400 cycles while charging for less than 15 min. I don’t have time this week to research and detail out all of the solid-state companies/systems and their progress toward this goal (future topic), but I will highlight one example for reference. Quantumscape released this white paper recently to discuss their progress with their cell performance to the public. This is promising because they seemingly hit the target for discharge energy over 400 cycles while charging in less than 10min.
This is progress, but the issue is that these are single layer “test” cells which is important to keep in mind when evaluating results or claims from companies. They are not ready to be produced and put into EVs anytime soon. The challenge of scaling up to multiple layers is a key component to commercialization. People are keen to point this out and a healthy skepticism of any new development is always good.
The intercalation of lithium is the only reaction taking place in a battery in the ideal world. In reality, since these are fairly complex systems with several materials, there are small reactions that take place where materials come in contact with each other. Fundamentally, solid electrolytes need to be ionic conductors of lithium (let lithium go through it easily). At first this was difficult at room temperature, but several material candidates have been identified to solve this issue. The problem is there are issues between the interfaces of the cathode-electrolyte and anode-electrolyte which will require stable interface materials or coatings to overcome reduced conductivity through these regions [1]. This is an issue because ionic conductivity is affected by temperature. Practically, this means colder environments would likely see worse performing cells.
If working well, the biggest factor for solid-state batteries to overcome will be cost. Currently there is no functioning cell that can come close to LIBs we have today along this metric. They are used in certain niche applications such as pacemakers or wearable applications [2]. For a lithium metal battery with solid electrolyte, the anode is either anode-less or has a thin film of lithium metal at the anode. At scale, material costs become the largest factor. With the increasing cost of lithium used in the cathode as well as anode, in this case could be an even larger issue. It obviously remains to be seen whether these high input costs will remain by the time solid-state batteries are ready to come to market.
Manufacturing processes need to be considered as well. Is synthesis of these solid-state electrolytes economical at scale, do they use any rare materials, are any of the materials toxic or reactive? Sulfide electrolytes tend to be made through a process that uses harmful hydrogen sulfide gas and are often times not favorably stable in air [3]. Are these challenges addressed by Solid Power for example, where scalability may be hampered by the need for ventilation and inert atmospheres? They don’t seem to mind, so perhaps they have solved these safety considerations and use this material to their advantage. SSBs need to be mechanically sound so that they do not allow dendrites to penetrate and short the cell. They also need to be non-brittle and flexible enough for roll-to-roll manufacturing [4]. Air stability is an issue as many solid electrolytes react with moisture so a dry environment would be necessary for synthesis and handling. These additional safety and handling considerations could pose problems for scalability and cost.
So What?
A solid-state battery has the potential to completely take over the market for LIBs and deem them obsolete on one hand. On the other the tech could not achieve commercialization cost/functionality standards and be limited to niche applications. Reality is often between the extremes, and I see no reason to doubt that here. The ideal case is that the SSB is more energy and power dense than the current LIB, cheaper or comparable in terms of cost, and scalable. The pain points of long charge times and safety realistically could be achieved as long as the technology keeps developing. Cost is the final pain point which is less in the control of the solid electrolyte developers. Material considerations, supply chains, geopolitics, energy costs, etc. all play a role. A successful SSB will have fewer rare elements, so it is cheaper and easier to scale. The synthesis method for these materials will also have to be created through the scalability and sustainability lens as well.
The best product market fit for SSBs is electric vehicles and that’s really what they are being developed for. As far as timeline is concerned, they aren’t likely to see commercialization until 2025-2030. Automakers like Toyota have said that they will start appearing in vehicles in 2025, but for hybrid applications. Thats only three years from now and it is really a race for who can make a functional cell the soonest. Solid Power, Quantumscape, Solid Energy Systems, and Factorial Energy are American SSB companies with partnerships with large car companies already. These are key partnerships as most car companies have aggressive electrification goals over the next 10 years. Further product market fit would be other transportation sectors (energy/safety dependent), grid storage (more cost/safety dependent), and perhaps space**. Realistically with the ideal SSB, your phone would have much more power and lifespan all while the battery takes up less space.
Solid-state batteries are a truly exiting technology with a hyped and anticipated market adoption. With high expectations to solve the EV’s issues, I suspect anything less than a polished product is likely to disappoint. Don’t forget researchers and companies have been developing LIBs for decades, not just years. While solid-state batteries are not a magic genie in a bottle that will solve our climate impact, they have the potential to solve the key pain points for EV batteries in the medium-long term (3+ years out). Long term safe and better functioning batteries, with less dangerous components would be a staple for an electrified vehicle future. Until next week,
-Grayson
* The specific capacity is 3861mAh/g for lithium, 3569mAh/g for silicon, and 372mAh/g for graphite. Ultimately the capacity is constrained by the cathode which has a specific capacity of ~170mAh/g (LFP) or even above 200 (NMC/NCA). Just enough anode material is required to store/convert enough lithium ions that the cathode can handle, too much extra would be a waste of space and resources. A higher capacity anode means that less material can be used, increasing overall energy density of the cell.
** In certain space applications temperatures get too cold, so thermal shields and a significant chunk of the energy goes into keeping the battery warm enough to function and so the liquid electrolyte doesn’t freeze. A SSB could potentially be better here without the liquid electrolyte to worry about. In deep space applications in which solar panels aren’t even practical let alone the batteries functioning, radioisotope thermoelectric generators are used for power.
Leave a like and let me know what you think!
If you haven’t already, follow me at twitter @graysonhoteling and check out my latest tweets.
Let someone know about Better Batteries and spread the word!
Socials
Twitter - @graysonhoteling
LinkedIn - Grayson Hoteling
Email - betterbatteries.substack@gmail.com
Archive - https://betterbatteries.substack.com
Subscribe to Better Batteries
Please like and comment to let me know what you think. Join me by signing up below.