The Perfect Battery? Pt. 2
Top 6 engineering challenges for Li-ion batteries
As I discussed last week, Li-ion batteries dominate as the energy storage/transfer mechanism of many aspects of our daily lives. In that post I discussed the top 6 chemistry challenges associated with making the perfect Li-ion batteries.
There is a lot that goes into making the commercially viable batteries that are in your phone or EV today. Last week was by no means a comprehensive list of even the parameters a chemist considers, but it intentionally left out engineering problems that companies face when developing out their products.
Without further ado, here are the top 6 engineering challenges to creating the best Li-ion batteries.
1) Volume:
Since the purpose of Li-ion batteries is to provide power for applications where a small mobile load provides the energy, it is important to get the volume as small as possible. Engineers don’t have a lot of room to work with so a large battery pack could mean the phone or laptop is too large for it to be feasible.
Similarly, every square meter of a car is precious space as you many know if you’ve ever packed up for a big trip. For this reason, electric vehicle engineers look for better packing techniques to limit the volume of space the batteries take up.
There are three different types of cells: cylindrical, prismatic, and pouch. Pouch cells offer the highest packing efficiency, but which style is used often depends on the application. There is a great overview of cell styles here.
2) Active material ratio:
Active materials are what are actually participating in the chemical reaction for Li-ion batteries to work. This includes the cathode, anode, and electrolyte (for an overview on Li-ion batteries and how they work click here). Unfortunately, there are inactive materials like current collectors, separator, additives, casing/shell that add precious weight and volume without adding more energy density.
This is like when you buy a turkey for thanksgiving, but it is filled with excess water and fat which drives up the per pound cost. Every useless material - those that don’t contribute to increasing functionality of the cell must be avoided at all costs.
Carbon black and polyvinylidene fluoride (PVDF) are often added to facilitate the cathode functionality and longevity. These materials are inactive components technically, so the less of these components the better. Researchers also trying to get lithium or silicon anodes to work instead of graphite which will reduce the overall volume of the cells and increase theoretical performance (future topic).
This is similar to the volume in 1), the goal is to get the most energy out of the least space. Here is an image of acetylene black (carbon), an additive that increases conductivity of the cathode.
3) Safety:
This one is pretty obvious, especially if you have paid attention to Li-ion batteries over the years. Samsung and Tesla have both had their criticisms for battery explosions and safety concerns. Each case is different and there are multiple destructive pathways.
If the separator fails or there is a manufacturing fault, a short circuit could occur. Thermal runaway is another issue where oxygen is formed in a detrimental side reaction and combination with an increase in heat can cause an explosion.
It is for this reason that engineers work diligently to make sure their cells are manufactured properly and set them up such that the heat may ventilate out of the batteries. This may be one of the most important factors for commercially viable batteries. A number of manufacturing issues were discovered with Samsung’s Galaxy Note 7 device. One of which can be seen below where the separator was penetrated causing a short circuit.
4) Cost:
The most expensive part of a Tesla car is the batteries. This may be the primary consideration because regardless of the other factors, if cost is too high, the cells won’t be bought and thus produced. Many things go into cost, but materials are the first consideration. In addition to humanitarian considerations and toxicity, cobalt is expensive so the less of it the better. Iron, manganese, nickel, lithium, copper, carbon, and aluminum are all elements commonly used in Li-ion batteries. The more abundant elements are typically cheaper because they are easier to mine from the ground.
Sodium can replace lithium at as the intercalating ion, but at a significant cut in energy for various reasons even though it would be much cheaper. It is possible we will see more Na-ion batteries in the future for cost considerations.
Another way to reduce costs would be to lower the manufacturing costs, updating cell designs, or standardizing the cells for mass production.
5) Functionality:
Weighing functionality vs. cost is ultimately what drives production. If the energy per dollar is worth purchasing to the consumer it will be produced. This drives battery engineers and researchers to do everything in their power to squeeze out every last Wh for in the cheapest possible way.
The functionality in large part comes down to the parameters discussed last week governed by material/element selection. Furthermore, materials and cell shape will depend largely on the application. Cars, phones, and satellites have different size, weight, temperature, and volume requirements.
In space temperatures can get very cold, and often much of the energy goes back into heating batteries so the electrolyte doesn’t freeze, whereas that is not a problem in terrestrial settings.
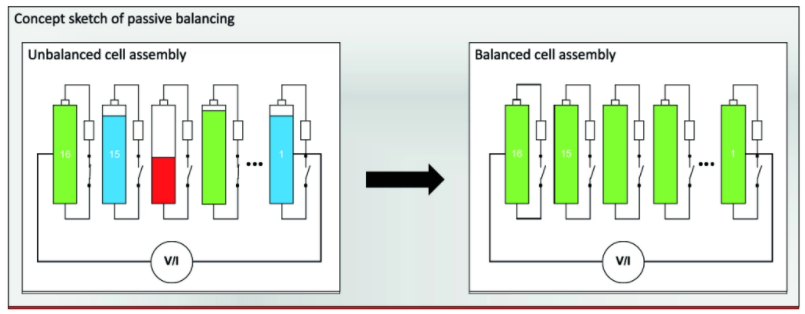
In addition, engineers may create battery management systems (BMS) that can control for heat and over/under charging. These conditions can hinder the performance and longevity of the cells. A proper system for maintaining efficiency and functionality of the cells once installed is an important factor. The BMS is also in charge of balancing charge levels in each cell to optimize performance and longevity as seen in the figure below from a article with a good overview.
6) Scalability:
For Li-ion batteries to take the next step and dominate the automobile and possible grid level storage, the production capability needs to be drastically increased. This means securing resources and supply chain, making sure shipping and material costs are reasonable. There may be an issue with certain material shortages if demand gets too high driving prices up, therefore battery companies using abundant materials or reliable suppliers will succeed.
It will also be important for industries to set standards so each company does not have to develop out custom cells. This would cut costs and allow more mass production. Scalability will be an important factor for any battery manufacturers. They will need to have equipment and new machines for producing large quantities of cells in a reliable way. Only when large scale production of Li-ion or similar batteries arise along with a reduction of costs will the wider adoption be able to take place.
Here’s a nice graphic that shows the manufacturing process that need to be optimized and scaled effectively.
I hope you enjoyed my list of top engineering challenges facing the Li-ion battery market! Don’t forget to subscribe for more great battery information!
-Grayson
If you haven’t already, follow me at twitter @graysonhoteling and check out my latest thread.
I’m also on LinkedIn. Please share this article anywhere you’d like with friends or followers!