If you’re reading this, I would really appreciate it if you pressed the heart button above :)
Battery Basics
Batteries don’t produce energy, they simply store it in the form of chemical energy. The energy must be produced somewhere else often times electricity. Batteries are split into primary and secondary categories. Primary batteries are non-rechargeable cells like AA batteries in your remote and some other niche types. Secondary batteries are rechargeable batteries like lead-acid, NiCd, NiMH, or Li-ion. Secondary batteries are the focus of this article. Secondary batteries are needed where there are applications that cannot have systems to create their own energy. This is typically limited to size and portability like phones, laptops, and satellites. The evolution of batteries has come from a desire to increase performance along two key parameters: energy density and power density. Power is the energy output per unit time which is important for applications that are very energy intensive, whereas energy density is the total energy that the battery can store which has more to do with runtime.
Parts/how it works
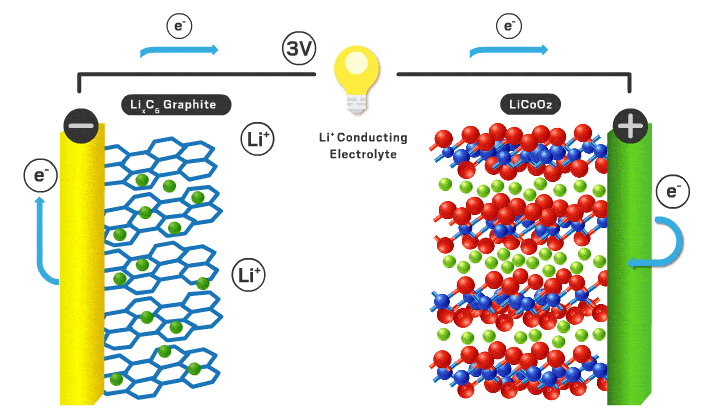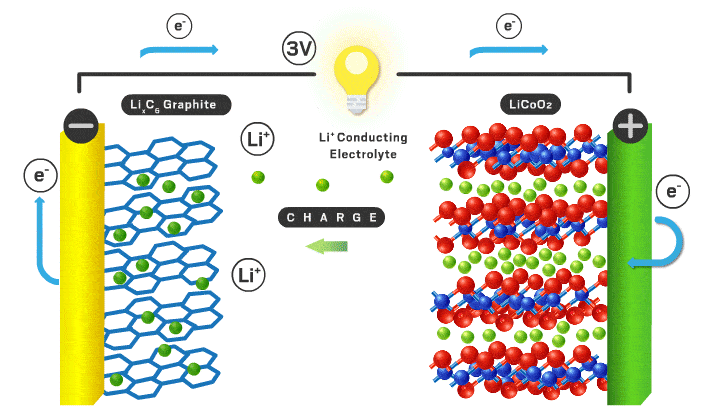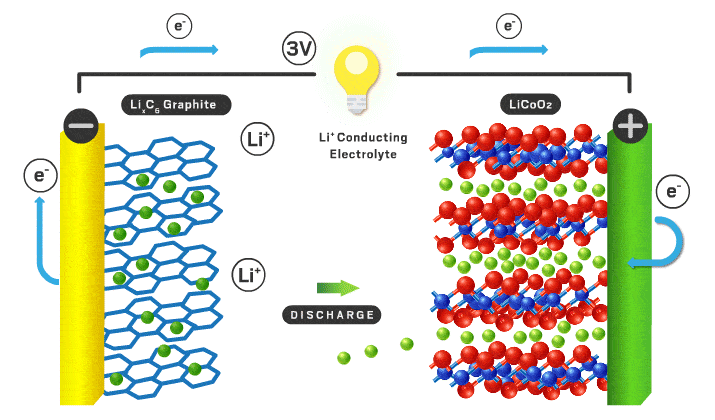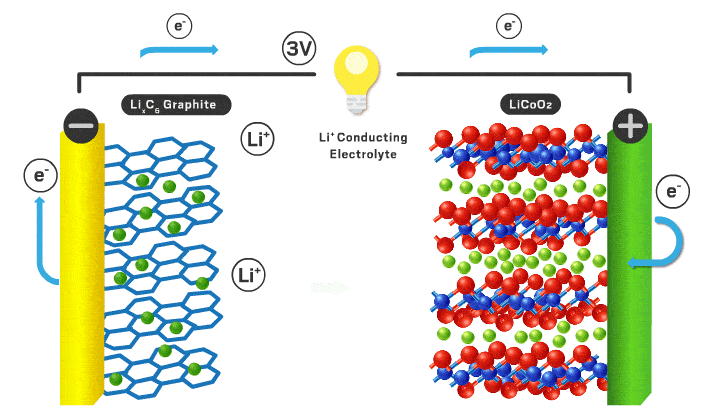
The parts of a Li-ion battery (LIB) include the cathode, anode, electrolyte, separator, and external circuit. Lithium ions are free to shuttle back and forth between electrodes (the positive cathode and negative anode). Lithium ions have a +1 charge and electrons have a -1 charge, so an electron is transported via an external circuit providing the electrical energy. The electrolyte is a medium for the lithium ions to travel back and forth easily. There is a separator to stop contact between the electrodes so a short circuit doesn’t occur. AKA the electrolyte/separator combo allows ionic conductivity without electronic conductivity. When fully charged all of the lithium ions are in the anode, but once it starts being used the lithium ions move to the cathode while the corresponding electrons flow through the external circuit turning stored chemical energy into electrical energy. To recharge an outside power source must supply the current in the opposite direction and the process is reversed.
Cathode
The cathode is the positive part of the cell and has been of most interest from researchers over the years. Cathodes need to be able to store lithium ions as well as have a stable structure when those ions leave when charged. Very few materials have made it to the commercial scale in LIBs. They need to conduct lithium ions and electrons effectively to be effective. In a commercial cell today the most common materials are lithium iron phosphate (LFP) or lithium metal oxides (NMC or NCA). LFP is less energy dense, but has less expensive precursor materials and is often safer. NMC stands for nickel, manganese, and cobalt. See You're putting that in my phone!? for supply chain implications of these battery materials. NCA stands for nickel, cobalt, and aluminum. Different combinations of transition metals effect different properties for the cathode for example voltage, reversibility, charge rate, safety, efficiency, etc. Graphite and PVDF are added to the active cathode material to increase electronic conductivity and act as a binding agent respectively. Lithium is the most important element in the mix as it is the chosen one to make the journey back and forth from cathode to anode. Sodium, potassium, calcium, and magnesium have the potential to be the intercalating ion like lithium but they all have their disadvantages like slower diffusion kinetics and less energy density.
Anode
The anode is the negative part of the cell and has historically been graphite. Anodes need to have excellent electronic conductivity as well as a stable structure able to house lithium ions. They should be durable, light weight, low cost, and have a voltage that matches the cathode/electrolyte combination. It needs to not react with the electrolyte to cause detrimental side reactions as well. Graphite does a good job at meeting all of these characteristics and is what is used today. Using lithium metal as the anode is of great interest because it would increase the overall energy density compared to a graphite alternative. The problem is there are undesired reactions that occur in the electrolyte that cause dendrites and short circuits typically. Silicon is another potential as it would also increase overall energy density, however structural stability in the form of volume changes are a concern.
Electrolyte
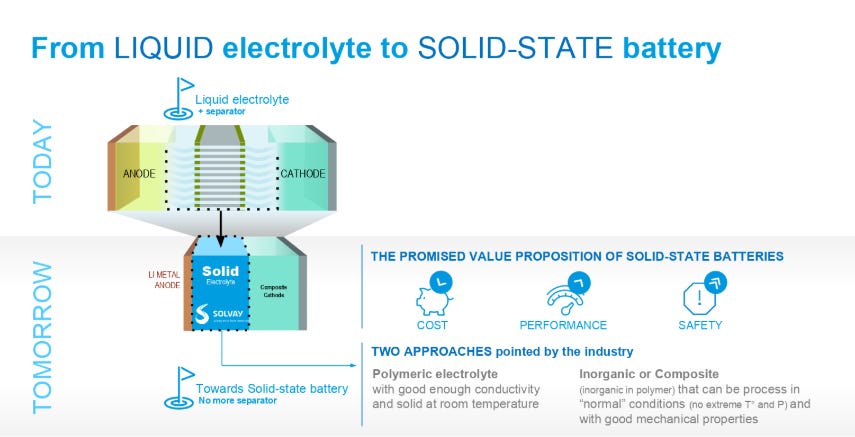
The electrolyte is a critical piece that makes everything possible. Typically this is an organic liquid solvent (ethylene carbonate or dimethyl carbonate), with a dissolved lithium salt (lithium hexafluorophosphate or lithium perchlorate). The material needs to freely allow lithium ions through. Since lithium can be dissolved in a liquid and ionic conductivity of liquids are typically better than solids, liquid solvents are used today. They need to have a voltage stability that matches the cathode and anodes both. If not, electrons will flow from the electrodes to the electrolyte to react rather than flow through the external circuit which we want. To allow for lithium metal or silicon anodes, a solid electrolyte is likely needed. Solid electrolytes also have better safety characteristics since the organic liquids used are flammable. The solid electrolyte needs to have very high ionic conductivity with very low electronic conductivity. This is a big chemistry challenge and materials have to be non-reactive in any other ways with the electrodes in addition to these vital parameters. Here is a quote from You're putting that in my phone!?
Two examples from growing companies include a sulfide based or ceramic solid electrolyte. Solids tend to have lower ionic conductivity than liquid by nature of the phase change, but these materials are designed to specifically conduct lithium ions. The best analogy I could come up with is swimming through water vs swimming through a foam/ball pit. There is no need for a separator with a solid state electrolyte as it serves the dual purpose of both.
Separator
The separator is only necessary with a liquid electrolyte as I mention above. The purpose of this material is to prevent contact between electrodes and stop electrons from being able to travel directly to the other electrode. This material needs to be porous so lithium ions in the liquid electrolyte can still pass through.
Production
Aluminum is used as the current collector for the cathode and copper for the anode. The production process is quite detailed and can be seen here. Steps include electrode manufacturing, cell assembly, and cell finishing. For electrode manufacturing, steps include mixing, coating, drying, calendaring, slitting, and vacuum drying. Cell assembly involves separation, stacking, packaging, electrolyte filling, winding, packaging, and electrolyte filing. The steps of cell finishing are then roll pressing, formation, degassing, aging, and EOL testing. As automated as this process has gotten, there are still a lot of steps, equipment, and materials needed to produce batteries.
Use Cases
It comes down to how much energy can be stored in the smallest volume. This is the determining factor for most applications and the product market fit Li-ion batteries have currently. This includes personal devices like laptops, tablets and phones. Other small devices, drones, military devices, and more use LIBs. As better batteries have come out, more energy intensive applications and longer battery lives became possible. Satellites can maintain orbit for much longer with LIBs and can use solar power when eclipsed. Furthermore electric vehicles are only possible with the large amount of energy LIBs provide such that they fit in the car without taking away any of the necessary space for the functioning car components. Miscellaneous use cases evolved to incorporate bikes, tools, backup power packs, and much more.
Issues
There are many chemistry challenges that go into making LIBs like reversibility, capacity, energy density, power density, capacity retention, and stability. Furthermore there are even more engineering challenges to producing commercially viable cells including volume, active material ratio, safety, cost, functionality, and scalability. LIBs are very complex arrangements of materials, with a complex set of synthesis/production steps, and a complex array of materials that come from around the globe which complicates supply chains. The cost of batteries in the future will actually depend on supply chain and mining of important metals among other things. Many of the materials are increasing in cost currently, are located in specific geographical locations worldwide, and may pose issues with such an increase in demand. Furthermore, China is the processor of the majority of battery materials posing a potential single point of failure for the supply chain.
So what
Last week I explained why I think why energy is the life force of human ingenuity and how batteries fit into that landscape. It is no doubt that batteries have made our lives exponentially better, sometimes in ways we don’t even realize or take for granted. The heroic journey of turning chaos into order is comparable to harnessing energy to overcome the disorder of our environment. Cheap abundant energy has made our world what it is today and it is important that we remember that batteries are an important mechanisms for storing our precious energy. Materials for batteries will be of vital national interest as we increase electric vehicle production and use intermittent wind/solar energy. Different chemistries, solid-state, flow batteries, and others could come into play in the future to solve issues that LIBs cannot achieve alone. The white house has aggressive plans for batteries and EVs over the coming years which is discussed in depth in this action plan and it is always worth knowing what they are up to. One last thing to mention is that there are many companies working to recycle batteries from EVs to phones which could be a big help to the battery economy. The battery industry is ever evolving and demand is going up fast while we wait for emerging technologies to hopefully come to fruition soon.
I hope you enjoyed this battery guide!
-Grayson
Leave a like and leave a comment on what you think!
Check out my latest tweets…


Let someone know about Better Batteries and spread the word!
Socials
Twitter - @graysonhoteling
LinkedIn - Grayson Hoteling
Email - betterbatteries.substack@gmail.com
Archive - https://betterbatteries.substack.com
Subscribe to Better Batteries
Please like and comment to let me know what you think. Join me by signing up below.