You're putting that in my phone!?
These are what materials are used in Li-ion batteries and what it means going forward.
Graphic: Chemical and Engineering News
Materials Matter
You may be lucky enough to not constantly think about the chemistry of processes in things when you see them like me. Oh look there’s Snell’s law of refraction, did you know why a curveball curves, oh look lithium ions are moving from cathode to anode when plugged in. Just like most people don’t care how they work, most people don’t know what materials are in your phone, laptop, or electric car battery.
This is actually a really important topic with increasing relevance because Li-ion battery demand increases with greater emphasis on EV production and net-zero standards. Li-ion battery materials need to be mined from the ground which is a labor intensive and very dirty process. They also are not evenly distributed throughout the world, so materials come from specific regions around the world. The materials in batteries play a crucial role in terms of humanitarian, economic, functionality, toxicity, and safety considerations.
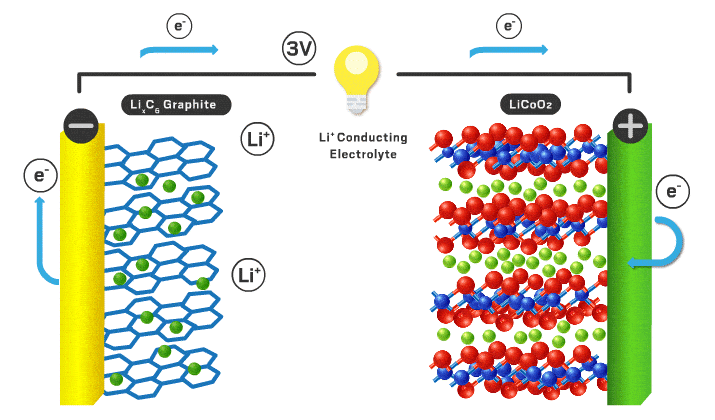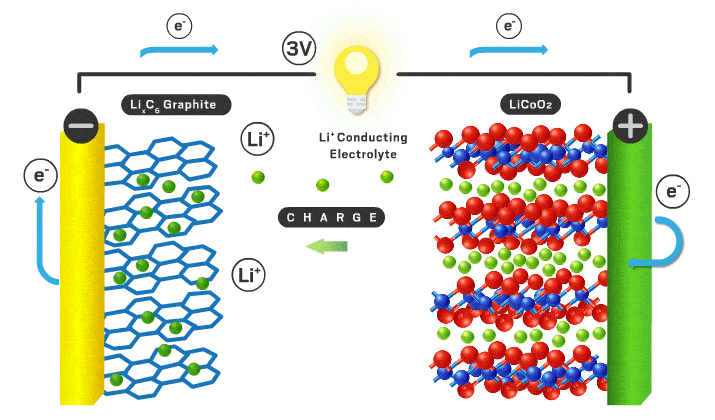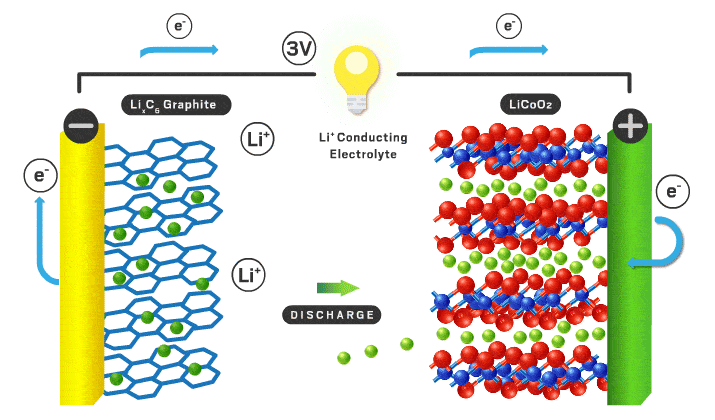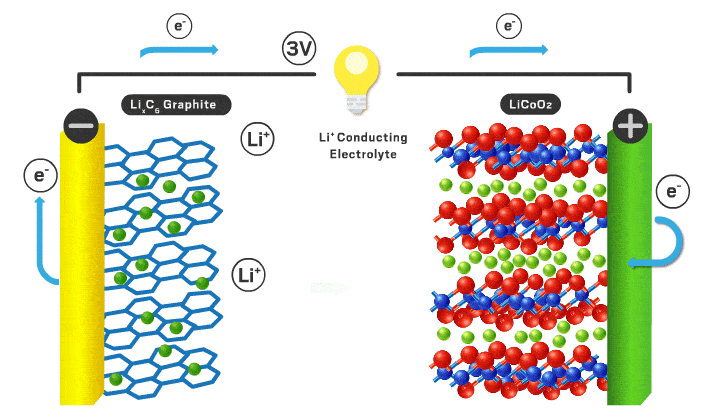
Each section will be a different part of the battery to make it more organized. Lithium ion batteries consist of a cathode, anode, electrolyte, separator, casing, and current collectors.
Cathode
Typical cathode materials include an intercalating ion, a transition metal, and negative ion.
Intercalating ion: This is the most important element and is typically lithium as it provides the most energy compared to the alternatives which include sodium, potassium, calcium, and magnesium. The reason the others do not work as well as lithium is because they have more atomic mass and are either larger or have a more positive charge. The former makes the gravimetric capacity* lower compared to lithium. Both of these latter properties make intercalation more difficult which causes a slew of other issues like efficiency, reversibility, and anode compatibility. The benefit of going to a different intercalating ion is abundance which is a driver for cost. Sodium is one of the most abundant elements and is the most likely alternative to lithium as demand is skyrocketing. Lithium is mined primarily in South America, not particularly abundant, but by no means is rare.
Transition metal: This is where much of the buzz is because cobalt works so well as a transition metal but everyone is trying to get rid of it due to humanitarian concerns, toxicity, and cost. Since it is toxic, production safety and proper disposal is an issue. Second and more importantly, the majority of cobalt is mined in the Congo which has terrible working conditions including child labor, terrible pay, and dangerous conditions. Nickel and manganese are the important metals for commercial cells today. Some applications use aluminum in the transition metal layer for stability. Research metals include vanadium, titanium, and niobium but these materials are not likely to gain much traction commercially. Changing the transition metal ratios even by slight margins has a huge impact on the properties and performance of batteries and thus where a lot of research time is devoted. One of the big challenges is making good cathodes without cobalt.
Negative Ion: Layered oxides NMC and NCA are the most common cathodes.** They both have a negative oxide ion to balance the charge and make up the structure. These materials are like a bookshelf or sandwich where there are alternating layers of lithium and transition metal layers which are all held together by oxygens. LFP* is the other most common cathode material which has the negative phosphate group instead of just oxygen. The main difference here is that phosphate groups give the material a completely different structural framework. Instead of a layered/sandwich type structure, LFP with its phosphate group has a more complex framework with channels or tunnels that the lithium ions move through.
Lithium, nickel, manganese, and cobalt are likely to be the most expensive and biggest supply issue in the future. These metals demand that new supply gets mined out of the earth which is a time and labor intensive process where geopolitical relationships can impact price and availability.
Anode
Current cells have a graphite anode, however companies are researching ways to use lithium metal or silicon instead.
Typically the anode is graphite which is just carbon atoms hexagonally in a layered orientation similar to the layered cathode materials I mentioned earlier. The lithium ions move into these layers when the battery is charged.
The only reason graphite is used as an anode is because lithium metal has traditionally failed as the anode. Before the mad rush for a solid state battery which may solve this problem, lithium metal anodes create “dendrites” or little fingers that build up and eventually cause a short circuit.
Silicon (not silicone or silica) is another promising anode candidate. This is promising as it offers a sizable increase in theoretical energy density and has space in the framework to hold lithium ions. The challenge here is that when lithiated, there is a volumetric expansion of the Si-Li material. This causes challenges of reversibility and energy density by which much research is carried out to solve with various methods.
Silicon is likely the future if the volume issue can be resolved in a simple and cheap way. Silicon is the most abundant element on earth as well which is a major plus for cost and supply chain considerations. Graphite is also easy to come by which means that current Li-ion battery manufacturers don’t have to worry about it. Currently the majority of graphite mining is located in China with other countries increasing production to meet demand.
Separator/electrolyte
In current commercial Li-ion battery cells, liquid electrolytes are predominant. These liquid electrolytes are an organic solvent (ethylene carbonate or dimethyl carbonate), with a dissolved lithium salt (lithium hexafluorophosphate or lithium perchlorate). The lithium ions in solution can travel back and forth from anode and cathode easily with high ionic conductivity (ions can move fast through the material, in this case the organic solvent). Various additives are usually added to help form a stable solid electrolyte interface (SEI)*** which can increase cycle lifespan.
With a liquid electrolyte, a separator is necessary to avoid contact between electrodes. It is typically a microporous polymer membrane such as polyethylene in which lithium ions can still pass through.
Emerging solid-state battery technologies will involve a solid electrolyte instead of a liquid. Two examples from growing companies include a sulfide based or ceramic solid electrolyte. Solids tend to have lower ionic conductivity than liquid by nature of the phase change, but these materials are designed to specifically conduct lithium ions. The best analogy I could come up with is swimming through water vs swimming through a foam/ball pit. There is no need for a separator with a solid state electrolyte as it serves the dual purpose of both.
Each company will have their own proprietary formula for their electrolyte. These materials will be constrained by raw material constraints just like that cathode, but perhaps to a lesser extent. It really depends on the formulations chosen.
Other
The current collector bridges the electrons at the electrode with external circuit to provide electrical energy. This is usually aluminum at the cathode and copper at the anode.
The casing around the cell can be an aluminum polymer laminate or nickel coated steel. All of these material are inexpensive and not likely a supply or cost bottleneck. Even at scale, copper, aluminum, steel, and polymer materials are already being used in industry.
Why This Matters
Why should you care about all this? Well first, the location of mining is a geopolitical risk factor and supply chain constraint. There is a push to use less cobalt in cells because of the working conditions for the Congolese people where most cobalt is mined. There is not enough pre-mined battery materials in supply so production is dependent on raw material mining around the world, often times in poorer countries around the world. The United States is not likely (or at least it will be faced with resistance) to mine these materials on its own soil for environmental considerations ironically enough.
Graphic: Visual Capitalist
Second is the rising price of these materials. With the extreme push for electric vehicles and carbon-zero energy standards using intermittent wind/solar energy sources, battery demand is going to increase drastically. Since the supply of lithium, nickel, manganese, and other raw materials is not as elastic as we would hope due to the physical limitations of extracting it from the earth, prices will increase. Even with the descending trend of Li-ion battery prices over the years, there has not been large scale demand like it will soon face. This will likely be an issue for the renewable energy movement as wind/solar will rely on Li-ion batteries to store energy during dark/calm seasons unless another energy storage solution can take its place.
A downstream consequence of switching to renewable energy in the face of increasing prices for such conversion, energy prices at large will increase. Increases in energy prices will mean more people unable to heat there homes and afford basic necessities. It is therefore vital to re-evaluate carbon-zero strategy/timeline estimations along with seriously considering the negative consequences. However, even if you support these policies and think it is worth it anyway, it will be important for battery manufacturers to secure raw material supply chain. They will have to focus on cheaper/more abundant raw materials and other energy storage chemistries will have to step up and solve the cheap energy storage problem as many companies/countries would simply not be able to afford the higher costs.
-Grayson
*Charge stored per unit of mass
*NMC=lithium [nickel manganese cobalt] oxide where the ratio of nickel, manganese and cobalt vary depending on the maker. Common ratio is one third of each transition metal. NCA= lithium [nickel cobalt aluminum] oxide. The ratio of these materials is usually 8:1:1. LFP is lithium iron phosphate.
***This is layer that forms over the anode and cathode respectively consisting of a new chemical structure. This layer needs to be allow lithium ions to still pass into the electrodes or the battery fails.
Thanks for reading, check out my latest twitter thread here.
Socials
Twitter - @graysonhoteling
LinkedIn - Grayson Hoteling
Email - betterbatteries.substack@gmail.com
Archive - https://betterbatteries.substack.com
Subscribe to Better Batteries
The best newsletter for learning more about batteries and the energy industry.
Please like and comment to let me know what you think. Join me by signing up below.